| Description |
(in progress) The “Droplet Digital PCR" (ddPCR) allows to: Detect small amounts of target DNA molecules with unparalleled sensitivity. Determine the change in the number of genomic copies (copy number variation) with extreme accuracy. Measure gene expression levels with extraordinary precision. Quantify NGS libraries. |
| Services provided | The Service supports the Institute's laboratories in experiments design, samples running and, if required, initial data analysis. |
| Equipment |
Biorad system "QX200 Droplet Digital PCR" Agilent Bioanalyzer 2100 |
| Contacts |
Dr. Elio Biffali Tel.: 081 5833298 e-mail: elio.biffali(at)szn.it |
| Description | The Real Time PCR service is equipped with a high productivity instrument which, using 384-well plates, not only allows to analyze a large number of samples in the same experiment, but also, by reducing the reaction volumes, to obtain a reduced cost for the single reaction. |
| Services provided | The Service supports the Institute's laboratories in experiments design, samples running and, if required, initial data analysis. |
| Equipment |
Life Technologies ViiA7 384 well-block Agilent Bioanalyzer 2100 |
| Contacts |
Dr. Elio Biffali Molecular Biology and Bioinformatics Tel. +39 081 5833298 e-mail: elio.biffali(at)szn.it |
2016
Santella L, Limatola N, Chun JT. (2016). The fertilization process: a new way to look at an old phenomenon. Atlas of Science: Another view on Science. Feb. 4.
Zhou C, Vitiello V, Casals E, Puntes VF, Iamunno F, Pellegrin, D, Changwen W, Benvenuto G, Buttino I. (2016). Toxicity of nickel in the marine calanoid copepod Acartia Tonsa: Nickel chloride versus nanoparticles. Acquatic Toxicology, 170: 1-12.
Percopo I, Ruggiero MV, Balzano S, Gourvil P, Lundholm N, Siano R, Tammilehto A, Vaulot D, Sarno D. (2016). Pseudo-nitzschia arctica sp. nov., a new cold-water cryptic Pseudo-nitzschia species within the P. pseudodelicatissima complex. Journal Phycology, 52:184-199.
Gallo A, Boni R, Buttino I, Tosti E. (2016). Spermiotoxicity of nickel nanoparticles in the marine invertebrate Ciona intestinalis (ascidians). Nanotoxicology, 12:1-9.
Boni R, Gallo ., Montanino M, Macina A, Tosti E. (2016). Dynamic Changes in the Sperm Quality of Mytilus galloprovincialis under continuous thermal stress. Molecular Reproduction & Development, 83:162-173.
2015
Santella L, Puppo A, Chun JT, Gragnaniello G, Garante E. (2015). Intracellular calcium increase and sperm incorporation following fertilization of a starfish egg. Nikon Small World in Motion Competition - Honorable Mention.
Limatola N, Chun JT, Kyozuka K, Santella L. (2015). Novel Ca2+ increases in the maturing oocytes of starfish during the germinal vesicle breakdown. Cell Calcium. 58:500-10.
Santella L, Limatola N, Chun JT. (2015). Calcium and actin in the saga of awakening oocytes. Biochem Biophys Res Commun 460:104-13.
Yazaki I, Tsurugaya T, Santella L, Chun JT, Amore G, Kusunoki S, Asada A, Togo T, Akasaka K. (2015). Ca²⁺ influx-linked protein kinase C activity regulates the β-catenin localization, micromere induction signalling and the oral-aboral axis formation in early sea urchin embryos. Zygote. 23:426-46.
Bosak S, Gligora Udovic , Sarno D. (2015). Morphological study of Chaetoceros wighamii Brightwell (Chaetocerotaceae, Bacillariophyta) from Lake Vrana, Croatia. De Gruyter, Acta Bot. Croat. 74 (2), X – X.
Santamaria G, Esposito CL, Cerchia L, Benvenuto G, Nanjappa D, Sarno D, Zingone A, De Franciscis V, Ribera d’Alcalà M. (2015) Aptamers are an innovative and promising tool for phytoplankton taxonomy and biodiversity research. Chemistry and Ecology.1-12.
2014
Chun JT, Limatola N, Vasilev F, Santella L. (2014). Early events of fertilization in sea urchin eggs are sensitive to actin-binding organic molecules. Biochem Biophys Res Commun. 450:1166-74.
Santella, L, Limatola N, Chun JT. (2014). Actin Cytoskeleton and Fertilization in starfish Eggs. In: Sexual Reproduction in Animals and Plants. Sawada H et al, eds. p. 141-153, ISBN: 978-4-431-54588-0, doi: 10.1007/978-4-431-54589-7-13.
Bosnjak I, Borra M, Iamunno F, Benvenuto G, Ujevic I, Buselic I, Roje-Busatto R, Mladineo I. (2014). Effect of bisphenol A on P-glycoprotein-mediated efflux and ultrastructure of the sea urchin embryo. Acquatic Toxicology, 156:21-29.
Liberti A, Melillo D, Zucchetti I, Natale L, Dishaw LJ, Litman, GW, De Santis R, Pinto MR. (2014). Expression of Ciona intestinalis variable region-containing chitin-binding proteins during development of the gastrointestinal tract and their role in host-microbe interactions. PLoS ONE 9: e94984.
| Description | Qualitative and quantitative analysis of nucleic acids by Agilent 2100 Bioanalyzer using a microfluidic chip, representing the gold standard for the QC of RNA samples to be used in sensitive applications such as qPCR, microarray, in situ hybridization, next generation sequencing, libraries preparation, etc. |
| Services provided |
Analysis of cDNA, total RNA, mRNA, RNA less than 200 nucleotides long (microRNA, siRNA, scRNA, snoRNAs, piRNA, 5S RNA, 5.8S rRNA). Qualitative and quantitative characterization by the use of: - NANO chip (available from Service) - PICO chip (provided by users) - "Small RNA chips" (provided by the user). |
| Equipment | Agilent Bioanalyzer 2100 |
| Contacts |
Dr. Elio Biffali Tel.: 081 5833298 e-mail: elio.biffali(at)szn.it |
 |
JEOL JSM-6700F Scanning Electron Microscope (SEM) To study cell surface morphology and composition |
 |
ZEISS LEO 912 AB Transmission Electron Microscope (TEM) To perform ultrastructural analysis of cells and tissues |
 |
ZEISS EVO MA LS Scanning Electron Microscope (ESEM) To analyze samples in their natural state |
 |
Microscopio confocale a scansione laser ZEISS LSM 700 Per l’osservazione in sezionamento ottico di campioni in fluorescenza, per l’analisi spettrale in fluorescenza |
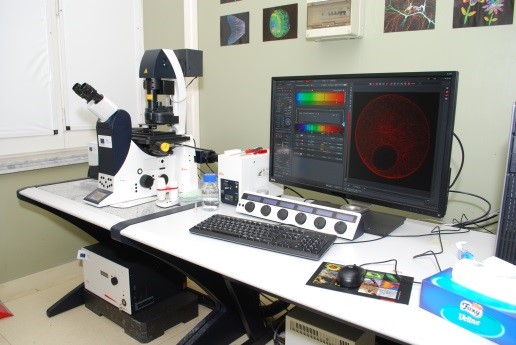 |
ZEISS LSM 700 Confocal Laser Scanning Microscope To perform optical sectioning of fluorescent samples and fluorescence spectral imaging |
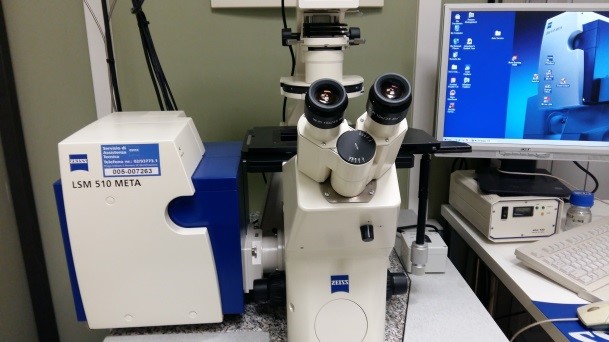 |
ZEISS LSM 510 Confocal Laser Scanning Microscope To perform optical sectioning of fluorescent samples |
 |
Imaging System LEICA DMI6000B |