Tenders and Job Opportunities
Tenders and Job Opportunities Job calls
Job calls Scholarships and research fellowships
Scholarships and research fellowships Open Calls
Open Calls ENGLISH
ENGLISH Pages
Pages
Pages
RESIGRASS
An holistic understanding of seagrass functioning and resilience to local-scale disturbances: from molecular to biogeographical scales
Summary
The physiological and ecological performance of ‘ecosystem engineers’ (e.g. seagrasses, kelps, corals) change from local to biogeographical scales, in response to contemporary and past processes. Understanding the resilience of ‘ecosystem engineers’ is particularly essential, because the type and number of human-induced disturbances has dramatically increased and global climate change is concurrently imposing high stress levels. The elements that contribute to the resilience of ‘ecosystem engineers’ are majorly unknown, and, therefore, represent a major challenge for modern ecology. The purpose of this proposal is to empirically assess whether genetic diversity, physiological versatility and ecological stability and resilience of an ‘ecosystem engineer’ (here, the seagrass Cymodocea nodosa) are connected from local to biogeographical scales. The implications of this proposal are relevant from a conservation perspective; if this study demonstrates that the resilience of species changes across the species’ distribution range, then conservation policies should be adapted to different regions according to the species capacity to overcome disturbances.
SZN role
Participant Institution involved in the genetic characterization of Cymodocea nodosa populations, and in the assessment of gene expression in controlled conditions.
Principal Investigator
Gabriele Procaccini
Project coordinator
Fernando Tuya Cortés (Universidad De Las Palmas De Gran Canaria)
Project lifetime
2016-2018
Funding Institution
Ministerio de Economia y Competitividad - Spain
Partners
Universidad De Las Palmas De Gran Canaria, Spain; Universidad De Las Islas Baleares, Spain; Stazione Zoologica Anton Dohrn, Italy
Completed projects
ABBaCo - Restauro Ambientale e Balneabilità del SIN Bagnoli-Coroglio
Approccio ecosistemico alla pesca ed acquacoltura sostenibile
COCONET - Towards COast to COast NETworks of marine protected areas (from the shore to the high and deep sea), coupled with sea-based wind energy potential
EMBRIC - European Marine Biological Research Infrastructure Cluster
HEATGRASS - Tolerance to HEAT stress induced by climate change in the seaGRASS Posidonia oceanica
RECCAM - Seagrass Meadows resilience to global warming: an analysis based on responses at ecophysiological, population and ecosystem levels
ABBaCo
Restauro Ambientale e Balneabilità del SIN Bagnoli-Coroglio

Summary
Dismissed industrial activities are responsible for persistent environmental degradation, mainly due to long-term accumulation of xenobitic contaminants in the environment. Such a chronic form of pollution represents a major threat for human health, biodiversity and ecosystem functioning. Necessary environmental remediation practices should however be coupled to restoration plans aiming at revert the degradation trend and give back healthy areas able to provide valuable ecosystem goods and services. Albeit fully integrated into the EU Restoration Agenda, marine environmental restoration is a new challenging issue in ecology, with Italy coordinating MERCES, the first European project in this field. The environmental restoration of Bagnoli-Coroglio Bay is a unique challenge at European level. ABBACO will develop new approaches for the removal and remediation of contaminated sediments and restoration of marine habitats. Actions include: i) identifying the environmental benchmark of the area; ii) assessing its present health status, iii) studying the effects of contaminated sediments on biodiversity and ecosystem functioning (MSFD), iv) assessing the combined effects of multiple stress at a hierarchical level; (v) experimenting innovative methods of transplantation and restoration of key species and habitats, and new biotechnological instruments for the remediation of sediments (bioremediation, bioaugmentation) in degraded habitats. ABBACO will provide novel expertise and stimulate new initiatives within the Blue Economy Agenda. The project results will be achieved by the actions of 6 intermingled work packages (WPs): WP1 Historic overview of the environmental status; WP2 Assessment of contamination and multiple environmental impact; WP3 Effects on biodiversity and ecosystem functioning; WP4 Holistic approach to the study of multiple stress and risk reduction; WP5 Pilot studies of restoration and rehabilitation; WP6 Evaluating the effects of restoration and rehabilitation procedures; WP0 Project management
What we do
We are coordinator of the project and play a key role in each of the 6 project’s WPs.
Partners
1) Stazione Zoologica Anton Dohrn, Napoli; 2) Istituto Superiore di Sanità (ISS); 3) Istituto Nazionale di Geofisica e Vulcanologia (INGV); 4-5) Consiglio Nazionale delle Ricerche (CNR-ISAC, CNR IAMC); 6) Agenzia Nazionale per le Nuove Tecnologie, l'Energia e lo Sviluppo Economico Sostenibile (ENEA); 7) Università Politecnica delle Marche; 8-9) Università degli Studi di Napoli Federico II (DiB, DICEA); 10) Università degli Studi della Campania “Luigi Vanvitelli”; 11) Università degli Studi di Napoli “Parthenope; 12) Consorzio Nazionale Interuniversitario per le Scienze del Mare (CoNISMa)
Research Area
Multidisciplinary Science
Project Lifetime
8th March 2017 to 7th March 2020
SZN Role
Coordinator
Principal Investigators
Luigi Musco, Vincenzo Saggiomo
Co-Principal Investigator
Lucia Rizzo
SZN people involved
Researchers: Bertocci I (WP4 co-leader), Bottaro M, Cardini U, Carotenuto Y, Castellano I, Costantini M, Crocetta F, D'Ambra I, Ferrante M, Hochscheid S, Locascio A, Marin Guirao L, Munari M, Pepi M, Rastelli E, Ristoratore F, Sansone C, Stefanni S, Zupo V
Experienced Researchers: Brunet C, Buia MC, Casotti R, Gambi MC, Iudicone D, Mazzocchi MG, Montresor M, Procaccini G (WP5 leader), Romano G, Sordino P, Spagnuolo A, Tosti E (WP4 co-leader)
Senior Researchers: Palumbo A, Ribera D’Alcalà M, Santella L, Zingone A (WP3 co-leader)
Associate Researchers: Badalamenti F, Vicinanza D, Vega Fernàndez T (WP1 leader)
Technologists: Conversano F, Margiotta F (WP3 co-leader), Patti FP, Sarno D, Terlizzi F, Toscano A, Saggiomo M
Experienced Technologists: Cirino P
Technicians: Cannavacciuolo M, Di Capua I, Lanzotti G, Passarelli A, Zazo G
Post doc: Gallo A, Guglielmo S, Morroni L (many others to be involved soon)
PhD Students: Dell’Anno F
Funding Institution
MIUR - Fondo Integrativo Speciale per la Ricerca (determina CIPE - GU n.56 8.3.2017)
Contribution to SZN: €2,000,000 (MIUR contribution) plus €1,700,000 (SZN co-financing contribution)
EvoCell
Animal evolution from a cell type perspective: multidisciplinary training in single-cell genomics, evo-devo and in science outreach
Summary
The aim of EvoCELL is to lay the foundation for a new branch of evo-devo focussing on cell types. We will study fundamental questions in animal evolution and development - eg. how new cell types arise in evolution, how many are in common between different animal groups and how many unique cell types have evolved in different animal lineages- using a new technology, single cell sequencing, which we will for the first time employ outside of lab models to sample the great diversity of animal phyla. EvoCELL will train a new generation of multidisciplinary scientists skilled in exploring the vast breadth of animal differentiation. We will jointly sample data from all major animal lineages, richly represented in the biodiversity of European waters, and develop new tools for comparative analyses, through which we will together pioneer three branches of cell evo-devo: evolution of stem cells; emergence of animal life cycles, and the stunning diversity of neural cell types. Through their excellent interdisciplinary and intersectoral training, from single-cell biology and palaeontology to bioinformatics and public outreach, our graduates will be in prime positions to assume leadership roles in academia, industry, and science outreach.
What we do
We are one of ten partners and are contributing to the diversity and evolution of neuronal cell types.
Partners
European Molecular Biology Laboratory, Heidelberg - DE; Stazione Zoologica Anton Dohrn, Napoli – IT; Uppsala University, Uppsala – SE; University of Heidelberg, Heidelberg – DE; University of Exeter, Exeter - UK; University College London, London – UK; Sars International Center for Marine Molecular Biology, Bergen – NO; Centre National de la Recherche Scientifique, Villefranches sur mer, Lion - FR; Non-academic partners: Museum für Naturkunde, Berlin – DE; Genomix4Life, Salerno - IT
Research Area
Organismal Biology
Project Lifetime
January 2018 to December 2022
SZN Role
Partner
Principal Investigator
People involved
2 ESRs to be hired
Funding Institution
European Commission, Horizon 2020 Call for Proposal: H2020-MSCA-ITN-2017. Grant Agrement no. 766053.
Contribution to SZN
€344081,76 (EU contribution)
Dedicated website
Media - Pictures

Meet the team
Maria I. Arnone, Researcher
Periklis Paganos, PhD student
Corbel
Coordinated Research Infrastructures Building Enduring Life-science Services
Summary
CORBEL is an initiative of thirteen new biological and medical research infrastructures (BMS RIs), which together will create a platform for harmonised user access to biological and medical technologies, biological samples and data services required by cutting-edge biomedical research. CORBEL will boost the efficiency, productivity and impact of European biomedical research. Individually, the services offered by the BMS RIs are critical to their own user communities. Collectively, through CORBEL, they will be transformative across the range of life-science disciplines: from generation of knowledge at the bench to patient treatment at the bedside.
What we do
The SZN participate to the WP4 (Community Driven Cross-Infrastructure joint research – Bioscience): Use case 4 (Marine Metazoan Developmental Models for BioMedical research - from predictive integrated databases to functional testing), coordinated by CNRS-Villefrances-sur-Mer (Evelyn Houliston). Within this WP the SZN hired for 18 mo the bioinformatician postdoc Elijah K. Lowe to work at enabling genomics and databases for Paracentrotus lividus.
Partners
Altogether, the CORBEL consortium comprises 37 individual partner institutions from 13 ESFRI Biological and Medical Sciences Research Infrastructures (BMS RI). Partners within WP4 are: EMBL-HD, EMBL-EBI, UMCU, ICFO, CRG, BRFAA, HMGU, CIRMMP, CSIC, CNRS, SZN, USTAN, FVB, MDC, VU/VUmc, DKFZ.
Research Area
Research Infrastructure
Project Lifetime
September 2015 to August 2019
SZN Role
Partner
Principal Investigator
People involved
Elijah K. Lowe - Salvatore D’Aniello - Anna Palumbo - Filomena Ristoratore
Funding Institution
European Commission Horizon 2020 research and innovation programme. Grant agreement No 654248.
Contribution to SZN
€90.700,00. (EU contribution)
Dedicated website
http://www.corbel-project.eu/home.html
Media - Pictures
Assemble+
Summary
ASSEMBLE Plus is a consortium of marine biological research stations in 16 countries, operating under the umbrella of the European Marine Biological Resource Centre (EMBRC-ERIC). The consortium provides scientists from academia, industry and government with transnational access to its marine biological research facilities, its historical data, and to advanced training opportunities, ASSEMBLE Plus aims to stimulate fundamental and applied research excellence in marine biology and ecology in Europe.
What we do
The 39 partner stations in the consortium provide access to a wide range of marine ecosystems, including the Red Sea, the Caribbean and Svalbard. We aim at attracting not only marine biological projects but also ones from non-marine sciences and from the private sector. We further aim to improve service provision by developing novel technologies and data solutions. Access to our stations is Trans-National, meaning that you can access ASSEMBLE Plus stations in any partner country other than the country in which you are employed.
If you wish to make use of this access program, you need to submit a short research proposal, and if this is selected, you will be able to carry out your proposed research at a marine partner station of your choice. The expenses of your stay as well as your travel costs will be covered, within certain limits, by ASSEMBLE Plus. If you are employed outside the EU, you can make use of the access program as well, but some restrictions apply.
For more information on ASSEMBLE Plus, including information on calls for proposals, rules, application- and access procedures, and submission deadlines, see http://www.assembleplus.eu. For any specific questions, contact Access Officer Florence Guillot; Email: access (at) embrc.eu
Partners
ASSEMBLE Plus Access Providers in the European Marine Biological Resource Centre (EMBRC-ERIC) are:
• EMBRC-Belgium (VLIZ, UGENT),
• EMBRC-France (Sorbonne Universités, CNRS),
• EMBRC-Greece (HCMR),
• EMBRC-Israel (HUJI),
• EMBRC-Italy (SZN, CNR),
• EMBRC-Norway (UiB),
• EMBRC-Portugal (CCMAR, IMAR, CIIMAR),
• EMBRC-Spain (UPV/EHU, UVIGO),
• EMBRC-UK (SAMS, USTAN, MBA, NERC-BAS, MSS),
ASSEMBLE Plus Access Providers not currently member of EMBRC-ERIC:
• Finland (UH),
• Germany (AWI, MPIMM),
• Ireland (NUIG),
• The Netherlands (NIOZ),
• Poland (IOPAN, UG-Gdansk),
• Slovenia (NIB),
• Sweden (UGOT – SLC).
Detailed information on the Access Providers is available on the ASSEMBLE Plus website at http://www.assembleplus.eu/
This distributed partnership provides access to diverse marine ecosystems and their biodiversity of the European coastal seas, the Red Sea (HUJI), the Caribbean (NIOZ), the Arctic (IOPAN) and the Antarctic (NERC-BAS).
Research Area
Research Infrastructure
Project Lifetime
48 months as of 1 October 2017
SZN Role
The SZN lead the Work Package “NA1 Improving Transnational Access (TA) provision”, coordinating the input from UPMC, UGOT, UPV/EHU, CCMAR, VLIZ, MSS.
The main objectives are:
• Establish a policy for regulating, granting and supporting TA;
• Set up single-access point for TA to the offered infrastructure;
• Test TA-pipelines through ASSEMBLE Plus and cognates in joint calls;
• Share best practices among platforms and services;
• Improve the efficiency of TA service provision.
SZN is involved as partner in three Joint Research Activities (JRAs).
JRA1 Genomics observatories
JRA3 Functional genomics
JRA4 Development of instrumentation
TA: In Italy, the SZN coordinates the transnational access to SZN in Naples and Ischia and to its Third Parties ISMAR-CNR in Venice http://www.ismar.cnr.it and IAMC-CNR in Messina http://www.iamc.cnr.it/index.php/contattaci/). All give access to Mediterranean pelagic and benthic hard- and soft bottom ecosystems.
SZN includes the main building in the Villa Comunale in Naples, the benthic ecology laboratory at Ischia Porto and the turtle research centre at Portici. Visitors have access to service platforms and research laboratories. SZN does not operate a guesthouse given ample supply of hotels and B&Bs nearby. SZN has extensive experience with collecting and maintaining model species and performing multi- disciplinary research on these organisms http://www.szn.it/index.php/en/research
Principal Investigator
Funding Institution
Université Sorbonne coordinates the project.
Contribution to SZN
€ 1.017.745,25 of which € 289.702,00 to the Third Parties
Dedicated website
2018 Events
 |
Migrazioni di mare: natura e cultura Martedì 11 dicembre 2018, ore 17.00 Introduce: Ulisse Cardini |
 |
Pensiero umano e intelligenza artificiale: relazioni pericolose? Martedì 6 novembre 2018, ore 17.00 Introduce: Maurizio Ribera d’Alcalà |
 |
Squali e miti: i mostri del Mediterraneo? Martedì 10 luglio ore 17.00 Sono intevenuti: Angelo Mojetta, Fabrizio Serena e Lorenzo Rossi |
 |
LA RICERCA E L'ITALIA: un rapporto difficile? Martedì 8 maggio ore 17.00 Introduce: Roberto Di Lauro Sono intervenuti: Roberto Defez, Andrea Graziosi, Tullio Jappelli, Roberto Di Lauro |
 |
Così vicini così lontani – l’uomo e gli altri Martedì 20 febbraio ore 17.00 Sono intervenuti: Emiliano Bruner, Elisabetta Visalberghi, Giancarlo Schirru |
 |
Presentazione del libro “Della Vecchiaia ovvero il posto delle fragole” di Domenico MazzulloMartedì 23 gennaio ore 17.00 |
2019 Events
 |
Presentazione del libro "Eco-Devo - Ambiente e Biologia dello sviluppo" Mercoledì 4 Settembre 2019, ore 17.00 Intervengono: Silvia Caianiello, Maurizio Casiraghi, Lorenzo Chiariotti |
 |
Un mare di plastica: come uscirne? Venerdì 24 maggio 2019, ore 17.00 Introduce: Domenico D’Alelio Intervengono: Mario Malinconico, Paolo Degiovanni, Alfonso Marino |
 |
Incontro con l'autore - La canzone del Guarracino Martedì 14 maggio 2019, ore 18.00 Ne parleranno: Chiara Arturo, Christiane Groeben, Eugenio Lucrezi e gli editori Lina Marigliano e Alberto D’Angelo Scena sonora con musiche originali di Tonino Taiuti |
 |
L’utilizzo della cinematografia nelle scienze Martedì 12 febbraio 2019, ore 16.30 Moderano: Anna Masecchia e Marcello Seregni Intervengono: Lorenzo Lorusso, Virgilio Tosi, Monica Zoppè Verrà presentato AA.VV., Osvaldo Polimanti e le origini della cinematografia scientifica, Carocci, Roma 2011, a cura di Lorenzo Lorusso, Virgilio Tosi, Giovanni Almadori |
 |
Internet e Democrazia Venerdì 11 gennaio 2019, ore 16.00 Intervengono: Guido Caldarelli, Rosanna De Rosa, Luciano Fasano
|
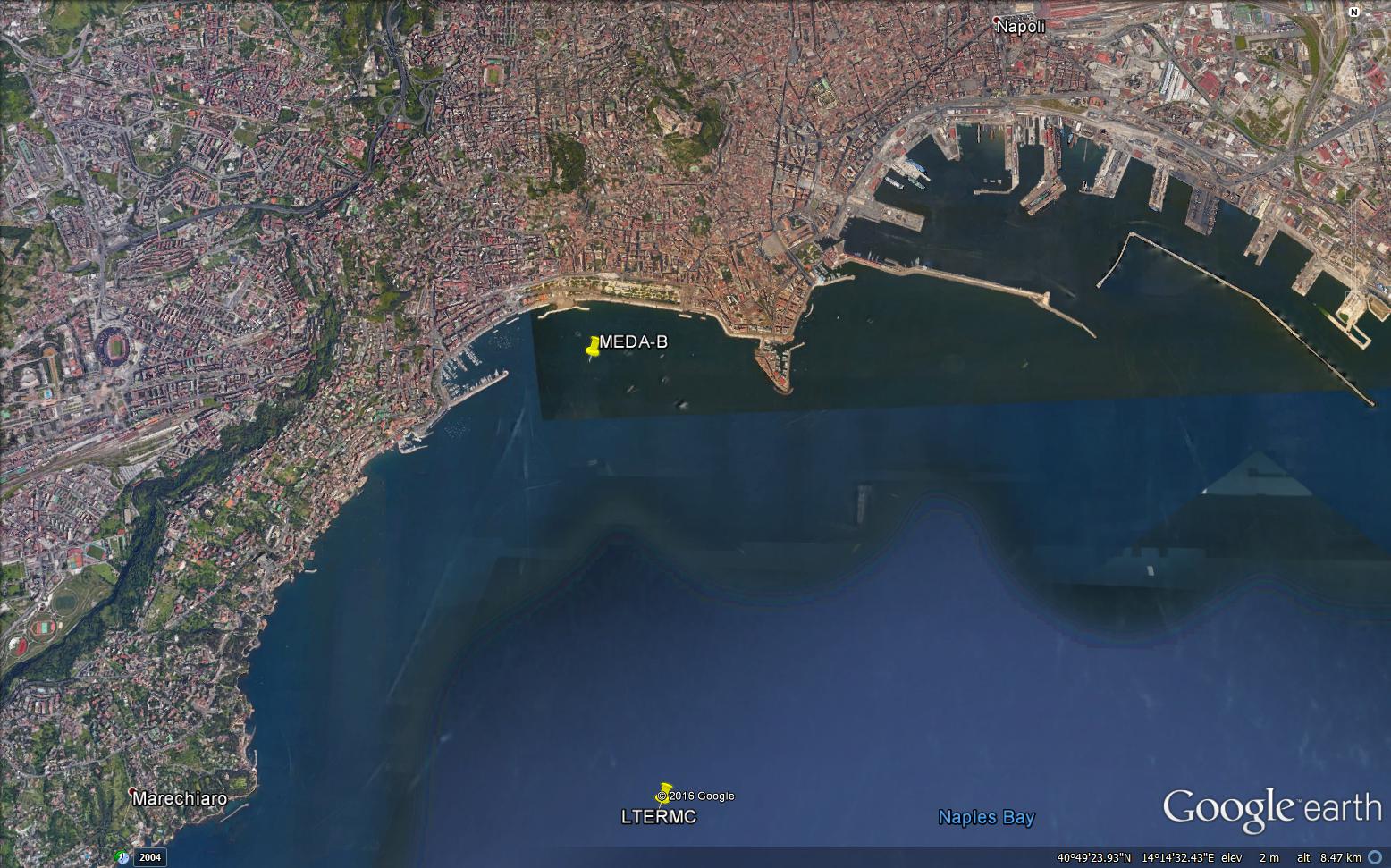
MEDA B Napoli
 |
 |
|
DATASET 2: ELASTIC BEACON MEDA B Napoli SIZE / DATA ARCHIVING MODE: .txt, .csv format with server archived data SZN DATA ACCESSIBILITY: currently not available online but available on request |
|
| Short Description | The elastic beacon "MEDA B Napoli" is located in the Gulf of Naples in front of Stazione Zoologica Anton Dohrn near the coast (Latitude: 40° 49.668' N, Longitude: 014° 13.984' E), at 17.5 m depth. The MEDA B is equipped with automatic instruments for continuous acquisition of weather-marine parameters, which allow to carry out high frequency and long-term measures. Data is transmitted in real-time to earth via a broadband Wi-Fi bridge and also via the GSM network. |
| Measuring Instruments |
Profile System (Multiparameter Probe) The system acquires the physical-chemical data along the water column from the surface to the depth of 15 meters. The parameters acquired are: Depth (m), Temperature (°C), Salinity (psu), Dissolved Oxygen (ml/l), pH, Fluorescence (RFU) and Irradiance (µE/m2sec) Weather sensors The weather station on MEDA acquires continuously and returns clockwise the following parameters: Wind Direction (Degrees), Wind Speed (m/s), Air Temperature (° C), Atmospheric Pressure (hPa), Humidity Relative (%), Rain (mm/h) and Photosynthetically Active Radiation (PAR μE/m2×sec) ADCP Current meter The ADCP is located on the bottom at a short distance from the MEDA continuously acquires and returns every 15 minutes the following parameters: Current Direction (Degrees), Current Speed (m/s), Wave Height (m), Wave Period (s), Wave Direction (Degrees), Tide (m) |
| Contacts |
Augusto Passarelli Monitoring and Environmental Data Unit (MEDA) tel. +39 081 5833 603-604 e-mail: augusto.passarelli(at)szn.it |