The Benthos and Nekton Ecology Functional Area is equipped with instruments useful for different types of analyses (biological, molecular, chemical and biochemical) and for the maintenance, in vivo, of animals, plant and algae.
The Benthos and Nekton Ecology Functional Area is equipped with instruments useful for different types of analyses (biological, molecular, chemical and biochemical) and for the maintenance, in vivo, of animals, plant and algae.
The Benthos and Nekton Ecology FA is located in:
- Ischia (MEDAS Centre)
- Naples (Villa Comunale)
At the moment, the Area of Naples is located in two rooms, waiting for the end of the renovation works of west wing:
- First floor room #346
- Ground floor room #16
|
Functional Area Manager Alice Mirasole This email address is being protected from spambots. You need JavaScript enabled to view it. Internal phone number #520 |
 |
Bookings on www.labagenda.com
 The Microbial Ecology area is dedicated to study and analyses of microorganisms with attention to the interactions between them and the environment.
The Microbial Ecology area is dedicated to study and analyses of microorganisms with attention to the interactions between them and the environment.
The area is temporarily distributed, pending the completion of the renovation works of the west wing, in the following rooms:
Ground floor: room #42-43
First floor east wing: room #138
Third floor east wing: room #MORGAN HALL
|
Functional Area Manager Anna Chira Trano This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Bookings on www.labagenda.com
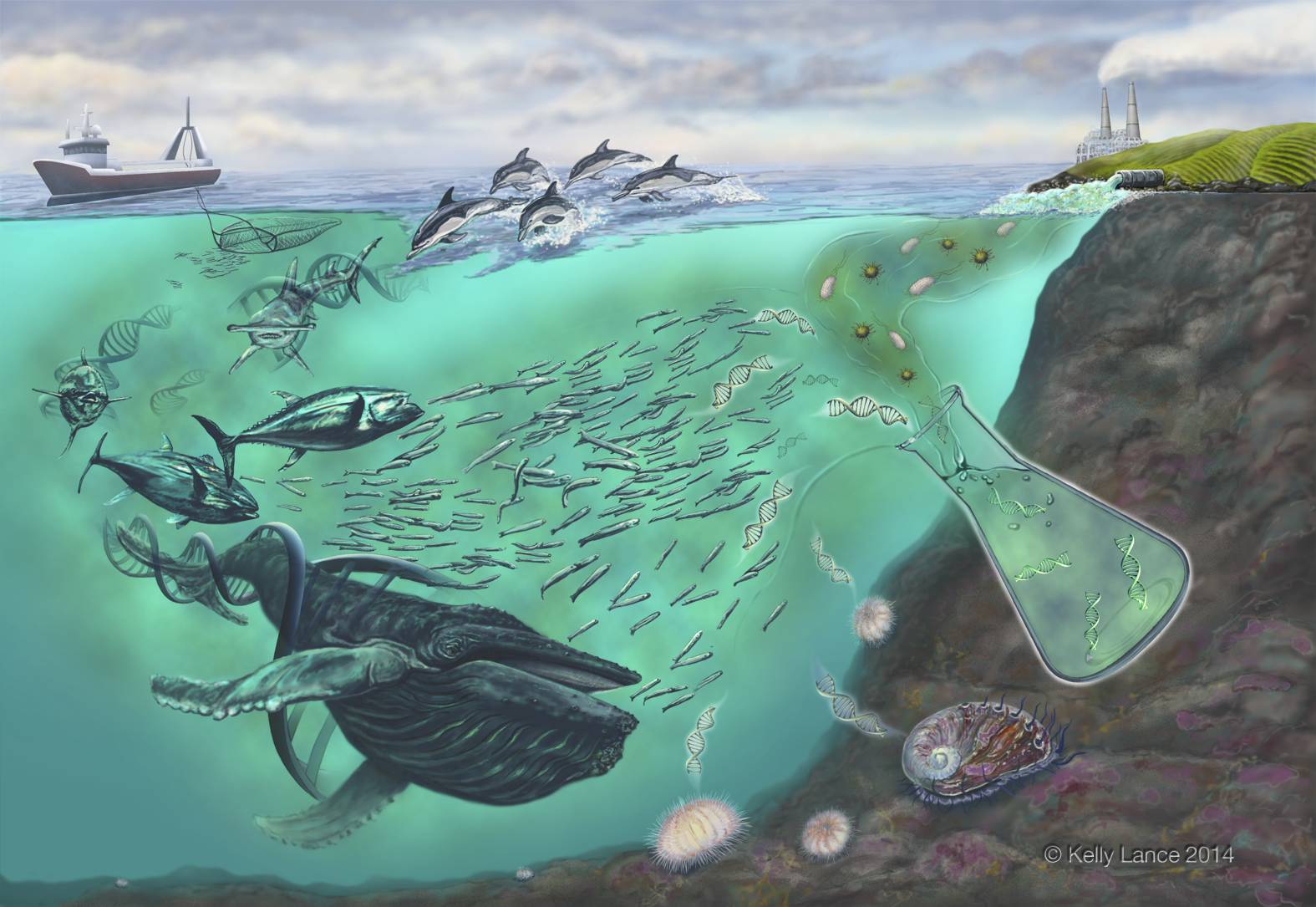 The Molecular Ecology Area is devoted to investigated molecular aspects related to taxonomic identification, population genetics studies, gene expression studies and functional genetics.
The Molecular Ecology Area is devoted to investigated molecular aspects related to taxonomic identification, population genetics studies, gene expression studies and functional genetics.
The area is temporarily distributed, pending the completion of the renovation works of the west wing in the following rooms:
- East wing, first floor rooms #140 and #150
- East wing, first floor room #146
- East wing, third floor room #340
- East wing, third floor room #346
|
Functional Area Manager Camilla Borgonuovo This email address is being protected from spambots. You need JavaScript enabled to view it. |
 |
Bookings on www.labagenda.com
 The Plankton Ecology Area is located at Naples centre. The Area allows users to analyze and study phytoplankton and zooplankton, from receiving sea samples, to isolating and culturing the species of interest, to allow their physiological study, population and genetics.
The Plankton Ecology Area is located at Naples centre. The Area allows users to analyze and study phytoplankton and zooplankton, from receiving sea samples, to isolating and culturing the species of interest, to allow their physiological study, population and genetics.
The area is temporarily distributed, pending the completion of the renovation works of the west wing, in 7 rooms and 2 common areas distributed on 4 of the 5 floors of the East wing, as listed below according to the activities carried out:
Wet Lab - Ground floor room #T35
Maintenance of phytoplankton and zooplankton cultures: First floor room #148 and third floor WALK IN cell #365ter
Live zooplankton manipulations - First floor room #138
Live phytoplankton manipulations – First floor rooms #138 and #148 and Third floor room #364
Fixed Phyto- and ZooPlankton manipulations - Third floor rooms #325 and #326
GROWTH CHAMBERS for EXPERIMENTS: floor -1 (along the wall in front of the stairs) and third floor (hall #365)
|
Functional Area Manager: Dott.ssa Carmen Minucci This email address is being protected from spambots. You need JavaScript enabled to view it. Internal Phone Number #272 |
 |
Bookings on www.labagenda.com
 The Biochemistry and Cell Biology area is equipped with various instruments useful for the biochemical and functional characterization of proteins and small molecules, including the study of signal transduction in marine organisms. The available equipment includes homogenizers for cell destruction, chromatographic systems for the purification and characterization of proteins, spectrophotometers for enzymatic assays.
The Biochemistry and Cell Biology area is equipped with various instruments useful for the biochemical and functional characterization of proteins and small molecules, including the study of signal transduction in marine organisms. The available equipment includes homogenizers for cell destruction, chromatographic systems for the purification and characterization of proteins, spectrophotometers for enzymatic assays.
 Manager: Andrea Sommella
Manager: Andrea Sommella
This email address is being protected from spambots. You need JavaScript enabled to view it.
Extension #228
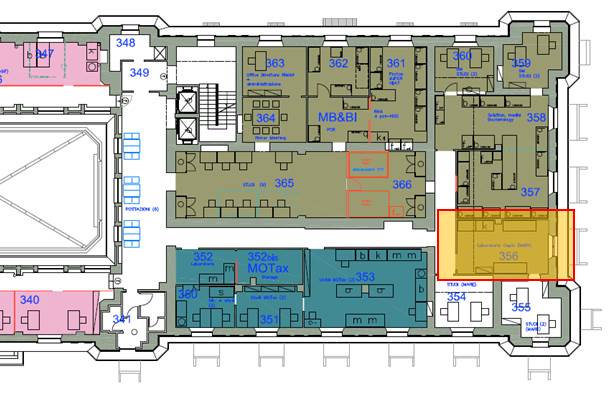
Third floor EST Wing, #361, 363, 365
| Bookable instruments |
| - Chromatographic System/FPLC Acta |
| - Spectrophotometer Agilent |
| - Spectrophotometer Agilent Cary 100 Scan |
| - Homogenizer ultraturrax Junke&Kunkel |
| - Incubator Thermo scientific |